Abstract
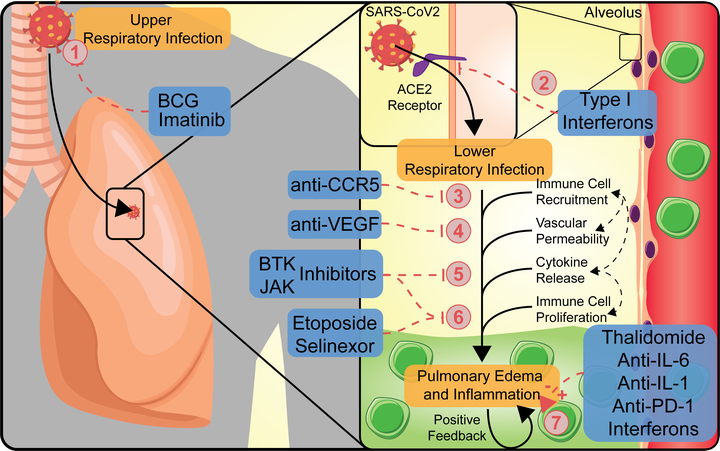
The novel coronavirus disease 2019 (COVID-19) pandemic has caused catastrophic damage to human life across the globe along with social and financial hardships. According to the Johns Hopkins University Coronavirus Resource Center, more than 41.3 million people worldwide have been infected, and more than 1,133,000 people have died as of October 22, 2020. At present, there is no available vaccine and a scarcity of efficacious therapies. However, there is tremendous ongoing effort towards identifying effective drugs and developing novel vaccines. Early data from Adaptive COVID-19 Treatment Trials (ACTT) sponsored by the National Institute of Allergy and Infectious Diseases (NIAID) and compassionate use study have shown promise for remdesivir, leading to emergency authorization by the Food and Drug Administration (FDA) for treatment of hospitalized COVID-19 patients. However, several randomized studies have now shown no benefit or increased adverse events associated with remdesivir treatment. Drug development is a time-intensive process and requires extensive safety and efficacy evaluations. In contrast, drug repurposing is a time-saving and cost-effective drug discovery strategy geared towards using existing drugs instead of de novo drug discovery. Treatments for cancer and COVID-19 often have similar goals of controlling inflammation, inhibiting cell division, and modulating the host microenvironment to control the disease. In this review, we focus on anti-cancer drugs that can potentially be repurposed for COVID-19 and are currently being tested in clinical trials.