Keeping Tumors in Check: A Mechanistic Review of Clinical Response and Resistance to Immune Checkpoint Blockade in Cancer
Abstract
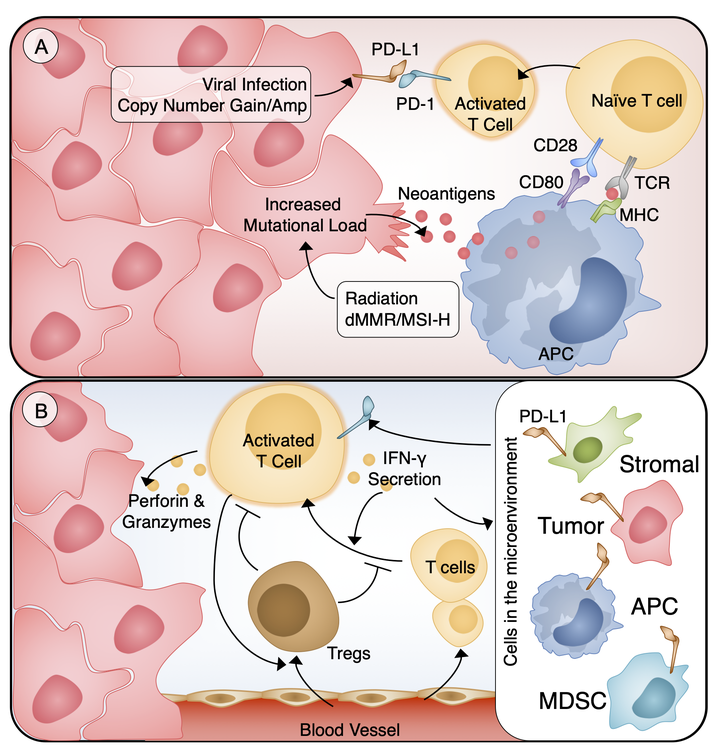
Immune checkpoints are a diverse set of inhibitory signals to the immune system that play a functional role in adaptive immune response and self-tolerance. Dysregulation of these pathways is a vital mechanism in the avoidance of immune destruction by tumor cells. Immune checkpoint blockade (ICB) refers to targeted strategies to disrupt the tumor co-opted immune suppression to enhance anti-tumor immunity. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death 1 (PD-1) are two immune checkpoints that have the widest range of antibody-based therapies. These therapies have gone from promising approaches to Food and Drug Administration-approved first- and second-line agents for a number of immunogenic cancers. The burgeoning investigations of ICB efficacy in blood and solid cancers have underscored the importance of identifying the predictors of response and resistance to ICB. Identification of response correlates is made complicated by the observations of mixed reactions, or different responses in multiple lesions from the same patient, and delayed responses that can occur over a year after the induction therapy. Factors that can influence response and resistance in ICB can illuminate underlying molecular mechanisms of immune activation and suppression. These same response predictors can guide the identification of patients who would benefit from ICB, reduce off-target immune-relate adverse events, and facilitate the use of combinatorial therapies to increase efficacy. Here we review the underlying principles of immune checkpoint therapy and results of single-agent ICB clinical trials, and summarize the predictors of response and resistance.